Scientists have developed a new approach that cuts the iridium needed for generating “green” hydrogen by 95%, while maintaining production rates. This could enhance the viability of a carbon-neutral hydrogen economy. Credit: SciTechDaily.com
A Japanese research team has made a breakthrough in hydrogen production, decreasing the necessity for iridium by 95% without compromising efficiency. This paves the way for sustainable, large-scale hydrogen energy solutions.
As the world shifts away from fossil fuels, many believe hydrogen will become the primary energy source. However, creating “green” hydrogen without using fossil fuels is challenging due to the need for iridium, a very rare metal.
In a study released today (May 9) in the journal, scientists led by Ryuhei Nakamura at the RIKEN Center for Sustainable Resource Science (CSRS) in Japan present a new method that reduces the iridium required for the reaction by 95%, without changing the rate of hydrogen production. This breakthrough has the potential to transform our ability to produce environmentally friendly hydrogen and facilitate the move towards a carbon-neutral hydrogen economy. Science, researchers led by Ryuhei Nakamura at the RIKEN Center for Sustainable Resource Science (CSRS) in Japan report a new method that reduces the amount of iridium needed for the reaction by 95%, without altering the rate of hydrogen production. This breakthrough could revolutionize our ability to produce ecologically friendly hydrogen and help usher in a carbon-neutral hydrogen economy.
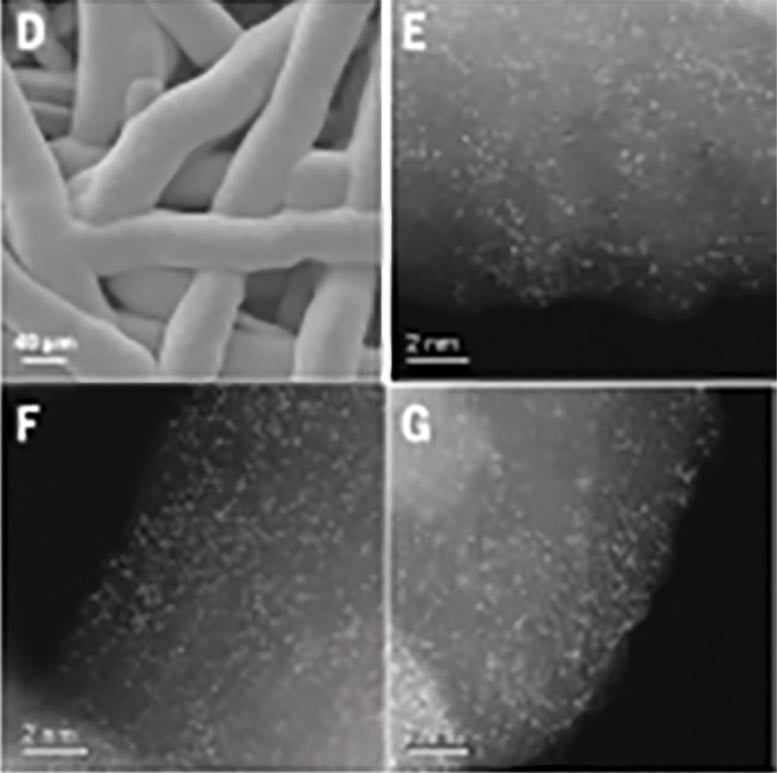
Scanning electron microscope image of the synthesized iridium oxide (D) and scanning transmission electron microscope images of the iridium (bright spots) dispersed on manganese oxide electrodeposited on a corrosion-resistant platinum-coated titanium mesh (E,F,G). Credit: RIKEN
Challenges in Hydrogen Production
Although 70% of the world is covered in water, extracting hydrogen from water on a scale that can compete with fossil fuels is not yet possible. To replace fossil fuels, alternative green energy production methods must match the current energy production rates.
The environmentally friendly method of extracting hydrogen from water involves an electrochemical reaction that requires a catalyst. The most effective catalysts for this reaction, which yield the highest and most stable hydrogen production, are rare metals, with iridium being the best. However, the scarcity of iridium poses a significant challenge. Co-first author Shuang Kong explains, “Iridium is so rare that scaling up global hydrogen production to the terawatt scale is estimated to require 40 years’ worth of iridium.”
Advances in Catalyst Development
The Biofunctional Catalyst Research Team at RIKEN CSRS is working to surpass the iridium bottleneck and discover alternative methods for producing hydrogen at high rates for extended periods. Ultimately, they aim to create new catalysts based on common earth metals, which would be highly sustainable. In fact, the team recently managed to stabilize green hydrogen production at a relatively high level using a form of manganese oxide as a catalyst. However, achieving industrial-scale production in this way is still years away. “We need a way to connect rare metal- and common metal-based electrolyzers, so that we can gradually shift over many years to completely sustainable green hydrogen,” says Nakamura. The current study accomplishes this by combining manganese with iridium. The researchers discovered that when they dispersed individual iridium atoms on a piece of manganese oxide so that they didn’t touch or clump with each other, hydrogen production in a proton exchange membrane (PEM) electrolyzer was sustained at the same rate as when using iridium alone, but with 95% less iridium. Potential and Future Directions
With the new catalyst, continuous hydrogen production was possible for over 3000 hours (about 4 months) at 82% efficiency without degradation. “The unexpected interaction between manganese oxide and iridium was key to our success,” says co-author Ailong Li. “This is because the iridium resulting from this interaction was in the rare and highly active +6 oxidation state.”
Nakamura believes that the level of hydrogen production achieved with the new catalyst has high potential for immediate usefulness. “We expect our catalyst to be easily transferred to real-world applications,” he says, “which will immediately increase the capacity of current PEM electrolyzers.”
The team has begun collaborating with partners in industry, who have already been able to improve on the initial iridium-manganese catalyst. Moving forward, the RIKEN CSRS researchers plan to continue investigating the specific chemical interaction between iridium and manganese oxide, with hopes of reducing the amount of necessary iridium even more. At the same time, they will continue collaborating with industrial partners, and plan on deploying and testing the new catalyst on an industrial scale in the near future.
Reference: “Atomically dispersed hexavalent iridium oxide from MnO2 reduction for oxygen evolution catalysis” 9 May 2024,
DOI: 10.1126/science.adg5193
A breakthrough in hydrogen production has been achieved by a Japanese research team, reducing the need for iridium by 95% without compromising efficiency, paving the… Science.
DOI: 10.1126/science.adg5193