A study has found two types of cell tightness that affect how cancer cells spread: one maintains cell groups by tissue tension and the other lets cells invade nearby tissues. This research reveals that the interaction between these tightness types is crucial in deciding if cancer cells can detach and become invasive, potentially increasing the risk of spreading. Source: SciTechDaily.com
The way cancer cells pull against one another decides if they can move to other parts of the body.
Understanding how cancer cells move from the original tumor is important for several reasons, including determining how aggressive the disease is. The movement of cells into the extracellular matrix (ECM) of neighboring tissue is a key step in cancer progression that directly relates to the start of spreading.
In a recently released paper in APL Bioengineering, published by AIP Publishing, a group of researchers from Germany and Spain used a breast cancer cell line panel and primary tumor explants from breast and cervical cancer patients to study two different cell tightness styles: one that creates collective tissue tension to keep cell groups together, and another, more directional, tightness that helps cells pull into the ECM.
“We focused on two aspects, the cells' ability to pull on the ECM fibers and create pulling forces, and their ability to pull on each other, causing high tissue tension,” explained author Eliane Blauth. “We associated each quality with different tightness mechanisms and examined how they are linked to cancer cell escape and tumor aggressiveness.”
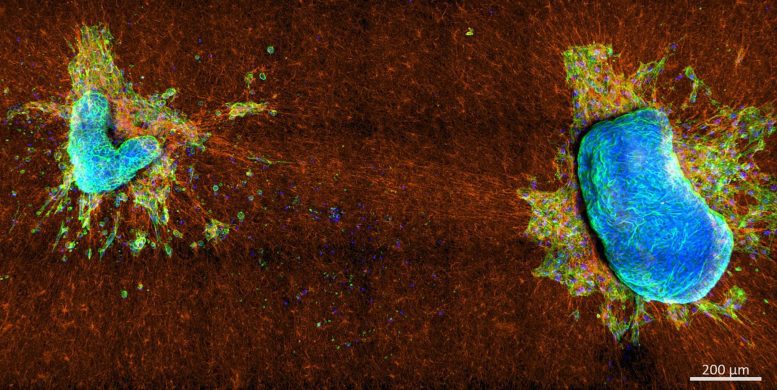
Two aggressive mixed mullerian tumor explants on a collagen network. Both tumor pieces attached to the collagen network and began pulling on the collagen fibers, causing extensive displacements and alignments, leading the cells with a dominant stress fiber-based tightness to escape. The distinct boundary structure of both pieces also indicates strong tissue tension, which prevents cell escape for cells with a dominant cortical tightness. Source: Steffen Grosser, Frank Sauer, and Eliane Blauth
The team discovered that more aggressive cells pull harder on the ECM than on themselves, while noninvasive cells pull harder on themselves than on the ECM. These different pulling behaviors are due to different structures of actin cytoskeleton inside the cells. Invasive cells mainly use actin stress fibers — thick actin bundles that spread across the cell — to create forces on their surroundings, while noninvasive cells generate forces through their actin cortex, a thin network just under the cell membrane.
The study revealed that it's not the total strength of these tightness styles, but the interaction between them, that decides a cell’s potential to escape. Experiments with moderately invasive cells showed the total force these cells apply on the ECM fibers is similar to that of noninvasive cells, yet they can still detach and invade the ECM, which noninvasive cells can't do.
“The noninvasive cells still have a high cortical contractility, keeping them together, while the moderately invasive cells have a nearly disappearing cortical contractility,” said Blauth. “So not much is holding them back even though they pull much weaker on the ECM fibers.”
The team’s measurements with patient-derived vital tumor explants confirmed their findings from the cell line experiments. Here, the number of cells with a high cortical contractility decreased during tumor progression.
“This further indicates that the ability of the cells to pull on each other and hold themselves clustered together becomes weaker as the tumor grows, potentially increasing metastasis risk.”
Reference: “Different contractility modes control cell escape from multicellular spheroids and tumor explants” by Eliane Blauth, Steffen Grosser, Frank Sauer, Mario Merkel, Hans Kubitschke, Enrico Warmt, Erik W. Morawetz, Philip Friedrich, Benjamin Wolf, Susanne Briest, Grit Gesine Ruth Hiller, Lars-Christian Horn, Bahriye Aktas and Josef A. Käs, 7 May 2024, APL Bioengineering.
DOI: 10.1063/5.0188186